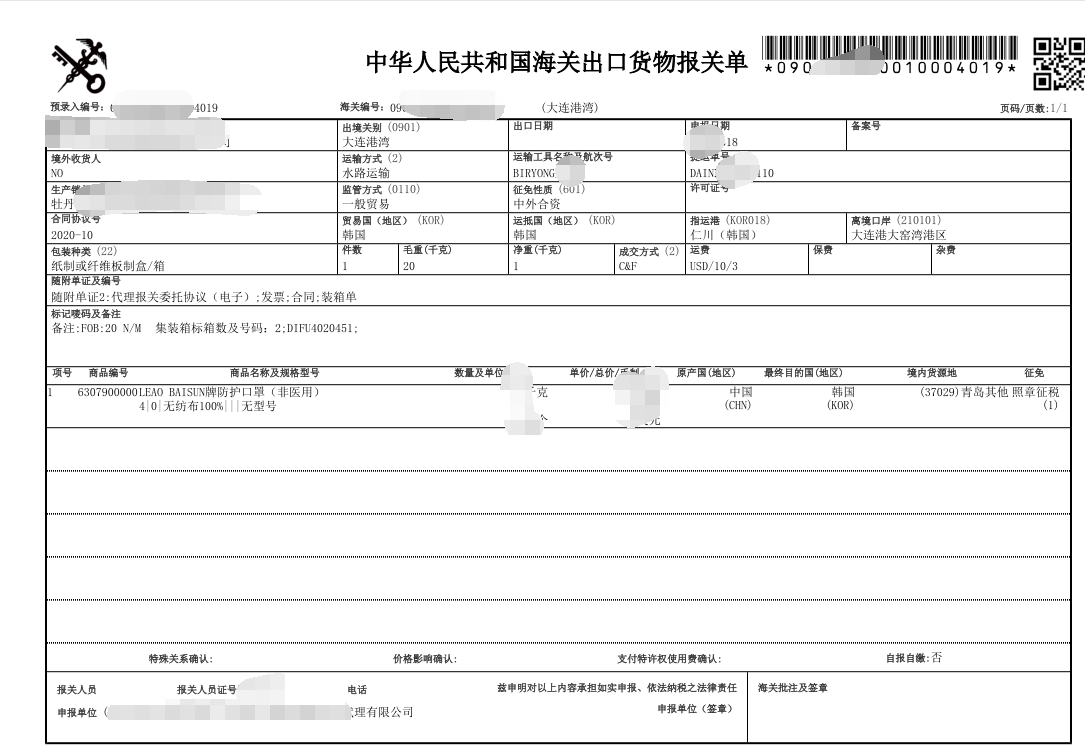
民用口罩出口报关流程
李国杰(经理):18202125732电话:021-61062636出口***物资的通关手续有什么变化?Whatarethechangesinthecustomsclearanceproceduresfortheexportofmedicalmaterials?答:“5号公告”发布以后,企业申报出口公告范围内的5类***物资时,在通关环节与之前相比需新增提交“***器械产品注册证书”和“出口***物资声明”,其中“***器械产品注册证书”应与报关单申报信息一致。海关凭***监督管理部门批准的***器械产品注册证书验放相关出口***物资。“53号公告”发布以后,有11类出口***物资需实施出口商品检验。此次新增实施出口商品检验的***物资不实施产地检验,申报时无需电子底账。企业需在申报时注明是否***。其中,在“5号公告”范围内的***物资,按照上述要求提交随附单据。其他出口***物资,建议企业按照《***器械分类目录》的管理类别,在向海关申报时,参照“5号公告”的要求,分别提供***器械产品注册/备案证明,以及质量安全声明。Answer:"afterannouncement5"isissued,whenenterprisesdeclare5typesofmedicalmaterialswithinthescopeofexportannouncement,theyneedtosubmit"medicaldeviceproductregistrationcertificate"and"exportmedicalmaterialdeclaration"inthecustomsclearancelinkcomparedwiththepreviousone.Amongthem,"medicaldeviceproductregistrationcertificate"shouldbec***istentwiththedeclarationinformationofthecustomsdeclaration.Thecustomsshallexamineandreleasetherelevantexportedmedicalmaterialswiththeregistrationcertificateofmedicaldevicesapprovedbythedrugregulatorydepartment.AftertheAnnouncementNo.53wasissued,11typesofexportedmedicalmaterialsneedtobesubjecttoexportcommodityinspection.Thenewlyaddedmedicalmaterialssubjecttoexportcommodityinspectionwillnotbesubjecttoorigininspection,andnoelectronicledgerisrequiredfordeclaration.Theenterpriseshallindicatewhetheritismedicalornotatthetimeofdeclaration.Amongthem,formedicalmaterialswithinthescopeof"announcement5",theattacheddocumentsshallbesubmittedaccordingtotheaboverequirements.Forotherexportedmedicalmaterials,itissuggestedthattheenterpriseshall,accordingtothemanagementcategoryoftheclassificationcatalogueofmedicaldevices,respectivelyprovidetheregistration/FilingCertificateofmedicaldeviceproductsandthequalityandsafetystatementaccordingtotherequirementsofannouncement5whendeclaringtothecustoms.“5号公告”和“53号公告”所列范围之外的物资出口,需要提交相关单证吗?Doyouneedtosubmitrelevantdocumentsfortheexportofmaterialsbeyondthescopeof"announcement5"and"announcement53"?答:对于公告范围之外的非***防护用品等出口物资,无需提交***器械产品注册/备案证明和质量安全声明,通关手续按原有要求办理。Answer:fornon-medicalprotectivearticlesandotherexportmaterialsbeyondthescopeofannouncement,itisnotnecessarytosubmittheregistration/FilingCertificateandqualitysafetystatementofmedicaldeviceproducts,andthecustomsclearanceproceduresshallbehandledaccordingtotheoriginalrequirements.李国杰(经理):18202125732电话:021-61062636专注于进出口的物流师在进出口清关的过程中遇到任何问题,都可咨询我,免费为您提供***解答。15年***进口清关团队竭诚为您服务上海|宁波|昆山|深圳|广州|厦门|成都|天津|青岛|北京|大连|***网点服务Intheprocessofimportcustomsclearance,youcanc***ultmeandprovideyouwithprofessionalanswersfreeofcharge.15yearsofprofessionalimportcustomsclearanceteamdedicatedtoserveyouShanghai,Ningbo,Kunshan,Shenzhen,Guangzhou,Xiamen,Chengdu,Tianjin,Qingdao,Beijing,Dalian,nationalnetworkservice)
